Asthma Treatment Guidelines 2020 is aimed [Ref]:
- To achieve good control of symptoms and maintain normal activity
- To minimize the risk of asthma-related deaths, asthma exacerbations, airflow limitation, and side effects of the medications
This is a simplified version of the 2020 asthma treatment guideline by GINA. For more details, visit the GINA Full Report.
Asthma management should be personalized and take into account the patient's preferences, allergen exposure and risk management, adverse effects of the medications, and comorbidities. Asthma severity assessment must be part of the treatment plan. Different severity assessment tools are available. One such tool is the “Consensus-based GINA symptom, Control Tool”
GINA Assessment of Asthma Control:
Asthma Symptoms Control |
Level of Asthma symptoms Control |
|||
| In the past four weeks, has the patient had: | Well-controlled | Partly Controlled | Uncontrolled | |
| Day time symptoms more than twice a week |
|
None of these symptoms | 1 – 2 of these symptoms | 3 – 4 of these symptoms |
| Night waking due to asthma |
|
|||
| SABA-reliever use for more than twice a week |
|
|||
| Any activity limitation due to Asthma |
|
|||
The correlation between symptom control and asthma exacerbation is poor. Some patients have fewer symptoms but continue to develop asthma exacerbations.
Specifically, elderly patients may have a poor awareness of airflow limitation, which could be masked by less physical activity and attributing the symptoms to comorbid conditions.
Some studies have evaluated the role of sputum eosinophilia, IgE levels, and FeNO (Fractional excretion of exhaled Nitric Oxide). However, these tests are not available widely, less cost-effective, and do not provide mortality or morbidity benefits when compared to simple symptoms/ lung function-based asthma assessment tools.
Asthma Treatment Guidelines 2020 - Initial management for adults and adolescents:
-
Infrequent Asthma Symptoms (less than twice a month without any risk factor for asthma exacerbation):
- Preferred initial Treatment includes Low Dose ICS-Formoterol - As needed (Evidence B).
- Other Treatment Options are Low Dose ICS whenever SABA is taken in combination or with separate inhalers (Evidence B).
-
Asthma symptoms (or need for a reliever medicine twice a month or more):
- Low Dose ICS + as needed SABA (Evidence A)
- As needed low dose ICS-formoterol (Evidence A)
- Other options include daily LTRA (It is less effective than ICS) (Evidence A) OR
- ICS whenever SABA is taken in combination or as separate inhalers (Evidence B)
-
Troublesome asthma symptoms most of the days or waking up at night due to asthma once a week or more (especially in the presence of risk factors):
- Low dose ICS-LABA as maintenance and reliever therapy with ICS-formoterol (Evidence A) OR
- Maintenance-Only ICS-LABA with as-needed SABA (Evidence A), OR
- Medium dose ICS with as-needed SABA (Evidence A)
-
Initial Asthma presentation is with severely uncontrolled asthma, or with acute exacerbation:
- Initiate regular controller treatment with a high dose of ICS (Evidence A), OR
- Medium dose ICS-LABA (Evidence D)
- Patients may need a short course of oral corticosteroids.
It is important to consider the following points in mind when initiating a controller treatment:
- Document/ Confirm the diagnosis of asthma.
- The patient's level of symptoms control, risk factors for asthma exacerbations, and baseline lung functions should be documented.
- Discuss available treatment options with the patients
- Emphasize treatment adherence and ensure a proper inhaler technique
- Schedule a follow-up visit to reassess the patient.
After controller treatment is initiated, depending on the clinical urgency, review the patient's response after 2 - 3 months. Review medication side effects, lung functions, and step-down therapy once good control has been maintained for 3 months or more. Summary of Step-Up Therapy - Asthma Treatment Guideline 2020 in Adults and adolescents twelve years of age or older: [caption id="attachment_21249" align="aligncenter" width="600"]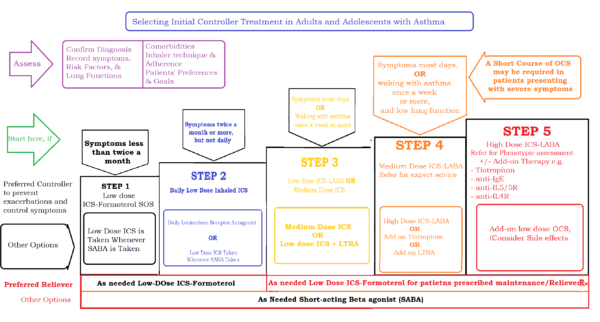 Summary of Step-Up Therapy - Asthma Treatment Guideline 2020 in Adults and adolescents twelve years of age or older[/caption]
Summary of Step-Up Therapy - Asthma Treatment Guideline 2020 in Adults and adolescents twelve years of age or older[/caption]
For a better visibility, a summary of steps 1 and step 2 are presented here in the image below:
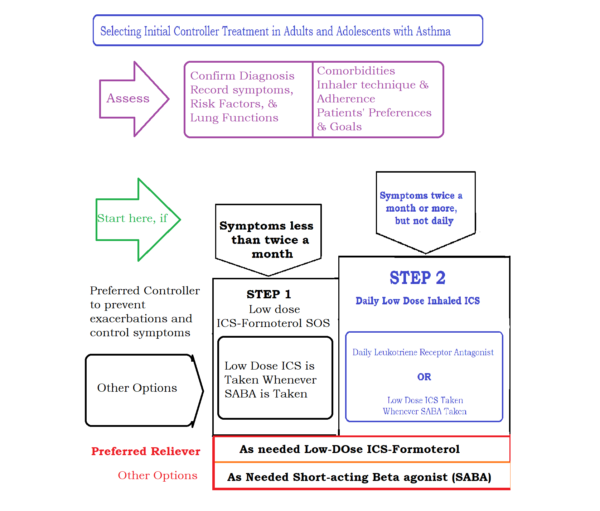
For a better visibility, a summary of steps 3 and above are presented here in the image below:
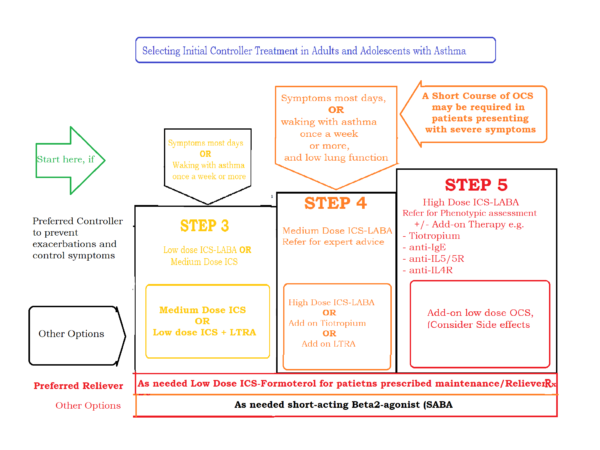
Asthma Treatment Guidelines 2020 - Initial management for children aged 6 - 11 years of age:
-
Infrequent Asthma Symptoms (less than twice a month without any risk factor for asthma exacerbation):
- Preferred initial Treatment includes As-needed SABA
- Other Treatment Options are Low Dose ICS whenever SABA is taken in combination or with separate inhalers.
-
Asthma symptoms (or need for a reliever medicine twice a month or more):
- Low Dose ICS + as needed SABA (Evidence A)
- Other options include daily LTRA (It is less effective than ICS) (Evidence A) OR
- ICS whenever SABA is taken in combination or as separate inhalers (Evidence B)
-
Troublesome asthma symptoms most of the days or waking up at night due to asthma once a week or more (especially in the presence of risk factors):
- Low dose ICS-LABA with as-needed SABA (Evidence A), OR
- Medium dose ICS with as-needed SABA (Evidence A)
- Other options include low dose ICS with daily LRTA, with as-needed SABA.
-
Initial Asthma presentation is with severely uncontrolled asthma, or with acute exacerbation:
- Initiate regular controller treatment with a medium dose of ICS-LABA with as-needed SABA. A short course of OCS may be needed.
- Other options include daily high-dose ICS-LABA, or add-on tiotropium, or add-on LRTA, with as-needed SABA
It is important to consider the following points in mind when initiating a controller treatment:
- Document/ Confirm the diagnosis of asthma, if possible
- The patient's level of symptoms control, risk factors for asthma exacerbations, and baseline lung functions should be documented.
- Discuss available treatment options with the patients
- Emphasize treatment adherence and ensure a proper inhaler technique
- Schedule a follow-up visit to reassess the patient.
After controller treatment is initiated, depending on the clinical urgency, review the patient's response after 2 - 3 months. Review medication side effects, lung functions, and step-down therapy once good control has been maintained for 3 months or more.
Suggested initial treatment in children aged 6 - 11 years of age with Asthma:

Suggested initial treatment in children aged 6 - 11 years of age with Asthma - Steps 1 and 2 (for better visibility):

Suggested initial treatment in children aged 6 - 11 years of age with Asthma - Step 3 and above (for better visibility):
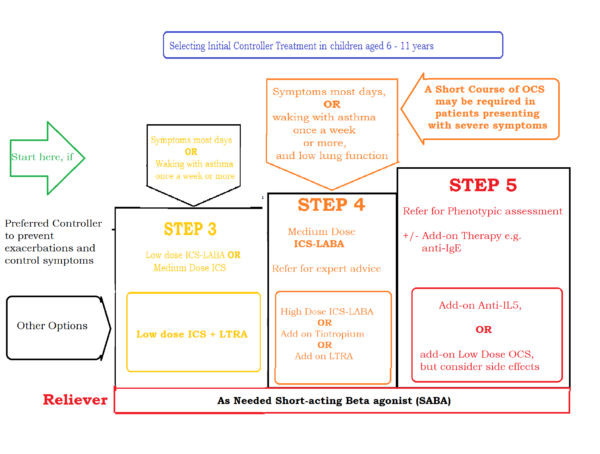
Table of inhalational corticosteroids - Dosages according to age groups:
|
Inhalational Corticosteroids Dose in adults and adolescents 12 years and older |
|||
|
Inhaled Corticosteroid |
Total Daily Dose in mcg |
||
|
Low Dose |
Medium Dose |
High Dose |
|
| Beclomethasone Dipropionate (standard particles) | 200-500 | >500 – 1000 | >1000 |
| Beclomethasone Dipropionate (extra fine particles) | 100-200 | >200 – 400 | >400 |
| Budesonide (DPI) | 200 - 400 | >400 – 800 | >800 |
| Ciclesonide (extra fine particles) | 80 - 160 | >160 – 320 | >320 |
| Fluticasone Furoate (DPI) | 100 | 100 | 200 |
| Fluticasone propionate (DPI) | 100-250 | >250 – 500 | >500 |
| Fluticasone propionate (pMDI, standard particles) | 100-250 | >250 – 500 | >500 |
| Mometasone Furoate (DPI) | 200 | 200 | 400 |
| Mometasone Furoate (pMDI, standard particle) | 200-400 | 200-400 | >400 |
|
Inhalational Corticosteroids Dose in children 6 to 11 years of age |
|||
| Beclomethasone Dipropionate (standard particles) | 100-200 | >200 – 400 | >400 |
| Beclomethasone Dipropionate (extra fine particles) | 50-100 | >100 – 200 | >200 |
| Budesonide (DPI) | 100-200 | >200 – 400 | >400 |
| Budesonide (nebules) | 250-500 | >500 – 1000 | >1000 |
| Ciclesonide (pMDI, extra-fine particles) | 80 | >80 – 160 | >160 |
| Fluticasone furoate (DPI) | 50 | NA | |
| Fluticasone propionate (DPI) | 50-100 | >100 – 200 | >200 |
| Fluticasone propionate (pMDI, standard particles) | 50-100 | >100 – 200 | >200 |
| Mometasone Furoate (pMDI, standard particles) | 100 | 200 | |
DPI (Dry Powder Inhaler), HFA (Hydrofluoroalkane propellent), ICS (Inhaled corticosteroids), NA (Not applicable), pMDI (pressurized metered-dose inhaler). ICS with pMDI should be preferably used with a spacer device.



 for multiple myeloma.webp)
.jpg)