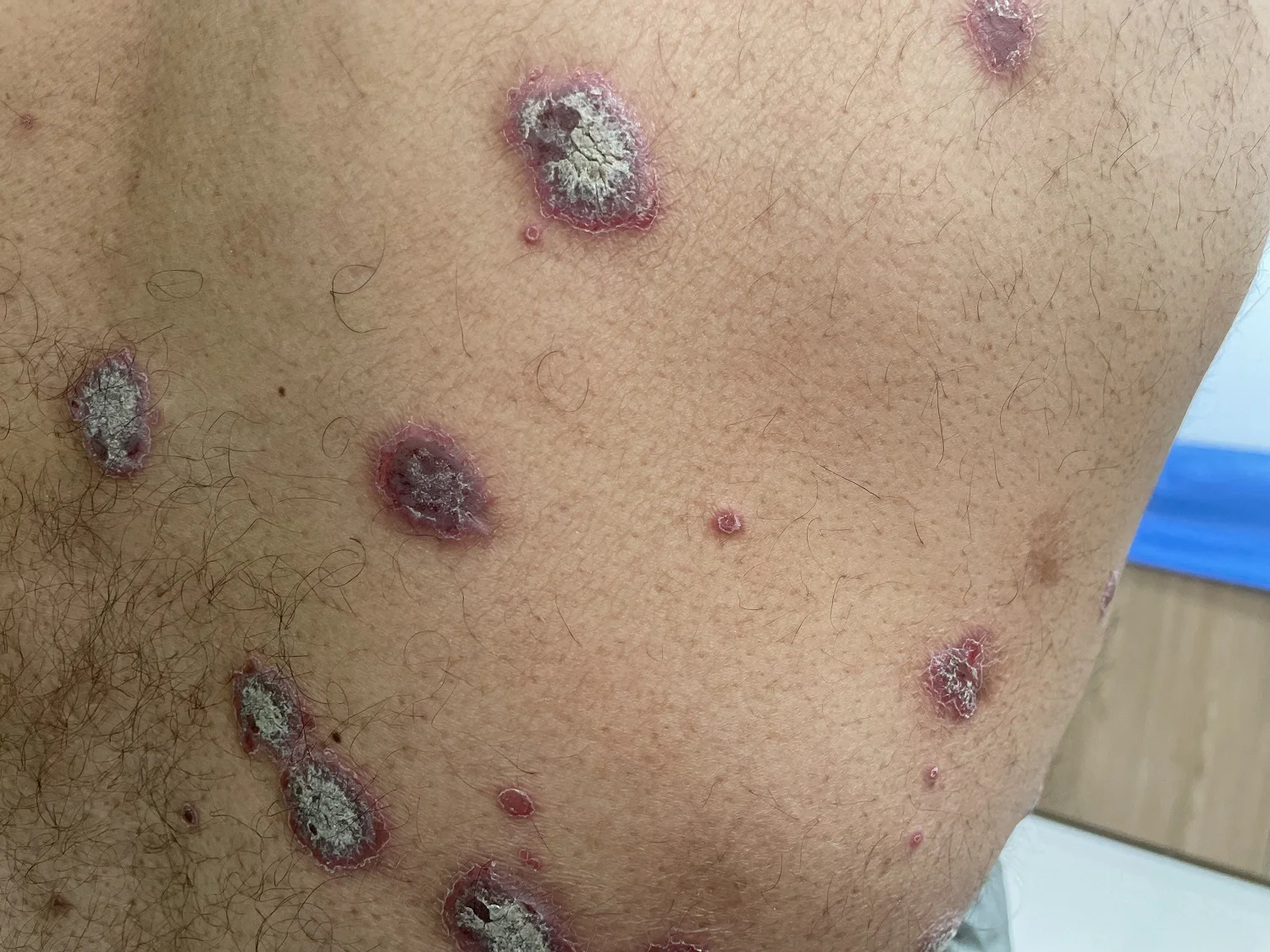
Plaque psoriasis is a chronic autoimmune skin condition characterized by the rapid growth of skin cells, leading to the formation of raised, red patches covered with thick, silvery scales. These plaques primarily appear on the elbows, knees, scalp, and lower back, though they can affect any part of the body.
Common symptoms include itching, burning, and pain, which can significantly impact personal comfort and daily functioning. The exact cause of plaque psoriasis remains unclear; however, it is believed to involve a combination of genetic, environmental, and immunological factors. The immune system mistakenly attacks healthy skin cells, causing inflammation and the accelerated growth of skin cells.
This skin disorder affects approximately 2-3% of the global population, making it a relatively common condition. It can occur at any age, with onset typically seen in early adulthood or later in life.
The presence of plaque psoriasis often leads to more than just physical discomfort; many patients experience psychological distress due to the visible nature of their condition. The social stigma associated with skin disorders can induce feelings of embarrassment, leading to social withdrawal and reduced quality of life.
The treatment landscape for plaque psoriasis has evolved over the years, encompassing topical therapies, phototherapy, and systemic medications. Initially, many patients relied on topical treatments such as corticosteroids and vitamin D analogs.
More advanced options have emerged, including conventional systemic therapies like methotrexate and biologics that specifically target pathways involved in the disease process. However, the heterogeneity of plaque psoriasis means that treatment effectiveness often varies from one individual to another, and limitations in the existing therapies highlight the pressing necessity for innovative treatment options like bimezelx. This new approval marks a promising advancement in the ongoing fight against moderate to severe plaque psoriasis, providing patients with additional hope for relief and improved quality of life.
Introduction to Bimezelx (Bimekizumab)
Bimezelx, known as bimekizumab, is a promising therapeutic agent recently approved by the FDA for the treatment of moderate to severe plaque psoriasis. This condition, characterized by red, inflamed patches of skin covered with silvery scales, affects millions of individuals worldwide.
Traditional treatments, such as topical therapies, phototherapy, and systemic medications, do not always provide adequate relief for all patients. The development of bimezelx represents a notable advancement in addressing the unmet needs within this patient population.
Bimezelx operates through a unique mechanism of action. It selectively targets and inhibits interleukin-23 (IL-23) and interleukin-17A (IL-17A), two cytokines that play a crucial role in the inflammatory processes associated with plaque psoriasis.
By modulating these pathways, bimezelx effectively reduces the inflammatory response, leading to improved skin clearance and overall patient wellbeing. This dual targeting differs from many existing therapies that tend to focus on a single pathway, thereby enhancing the potential for more effective management of the disease.
The development of bimezelx involved rigorous clinical trials, which have demonstrated its efficacy and safety profile. In phase 2 and phase 3 studies, participants experienced significant improvements in their psoriasis symptoms compared to placebo groups.
The results indicated not only reductions in psoriasis severity but also led to an improved quality of life for many individuals. Such outcomes have played a crucial role in facilitating FDA approval, marking a pivotal moment in treatment options available for plaque psoriasis.
As patients and healthcare providers seek innovative solutions, bimezelx stands out as a new contender in a burgeoning landscape of biological therapies designed to combat this chronic condition effectively.
Implications of FDA Approval of Bimezelx:
The recent approval of Bimezelx (bimekizumab) by the FDA marks a significant advancement in the treatment landscape for moderate to severe plaque psoriasis. This innovative therapy introduces a new mechanism of action targeting specific pathways involved in the inflammatory response associated with plaque psoriasis.
The approval is poised to provide considerable benefits to patients who have historically experienced limited success with traditional therapies. As a monoclonal antibody targeting interleukin 17A and interleukin 17F, Bimezelx demonstrates the potential for improved efficacy and safety profiles, thereby enhancing patient outcomes and overall quality of life.
For patients, the introduction of Bimezelx may address unmet needs, especially for those who have not achieved adequate control of their psoriasis with existing treatments. The reported clinical trial results indicate not only a greater rate of skin clearance but also a reduction in the severity of symptoms.
As a result, patients might experience fewer flare-ups and a significant improvement in the psychological and social burdens associated with chronic skin conditions. Improved outcomes can lead to enhanced daily functioning, increased self-esteem, and better overall well-being for individuals living with plaque psoriasis.
From the perspective of healthcare providers, the approval of Bimezelx requires a re-evaluation of prescribing practices and patient management strategies. With new options available, clinicians may need to assess the appropriateness of Bimezelx on a case-by-case basis, considering factors such as patient history and previous treatments.
Additionally, the introduction of this medication may necessitate further education and training for healthcare professionals to effectively incorporate it into treatment plans. Overall, the approval of Bimezelx represents a pivotal moment that may reshape therapeutic approaches and enhance the care provided to patients suffering from plaque psoriasis.
Future Outlook for Psoriasis Treatments
The landscape of psoriasis treatments is constantly evolving, driven by advancements in research and a growing understanding of the underlying mechanisms of chronic skin conditions. The recent FDA approval of Bimezelx (bimekizumab) for the treatment of moderate to severe plaque psoriasis represents a significant milestone in this evolution.
Bimezelx operates by targeting specific pathways involved in the inflammatory processes that underpin psoriasis, thus offering new hope for patients seeking effective management of their condition.
In the realm of ongoing research, scientists are exploring various therapeutic avenues, including biologic agents and small molecule inhibitors, that may further enhance treatment efficacy and safety profiles.
As our comprehension of the immune system and skin biology deepens, new biologics are emerging that target distinct inflammatory pathways, presenting opportunities for more individualized treatment plans tailored to each patient's unique disease manifestation. Bimezelx exemplifies this trend, highlighting a shift towards precision medicine in dermatology.
Additionally, the importance of patient-centered approaches cannot be overstated. As clinical trials continue to evaluate the long-term safety and effectiveness of novel treatments, such as Bimezelx, there's an increasing emphasis on developing therapies that address both the physical symptoms and the psychosocial impact of plaque psoriasis.
Future treatment paradigms will likely incorporate comprehensive patient assessments to ensure that therapy not only alleviates skin symptoms but also improves overall quality of life.
As we look ahead, the integration of technology in treatment monitoring and the development of algorithms for personalized treatment regimens may further revolutionize the care of those living with psoriasis.
Overall, the future of psoriasis treatments is promising, characterized by innovation, a focus on personalization, and an unwavering commitment to improving patient outcomes.