HIV prevention has taken a significant step forward as Gilead Sciences explores the potential of a once-yearly dosing option for lenacapavir.
After demonstrating impressive efficacy as a twice-yearly injection, the company is now preparing to initiate phase 3 trials for an annual regimen. Here’s what we know so far about this groundbreaking development.
Promising Results from Phase 1 Trials:
Recent phase 1 data presented at the Conference on Retroviruses and Opportunistic Infections, and published in The Lancet, revealed that two once-yearly formulations of Lenacapavir achieved blood concentrations well above the levels associated with strong HIV prevention efficacy in earlier studies.
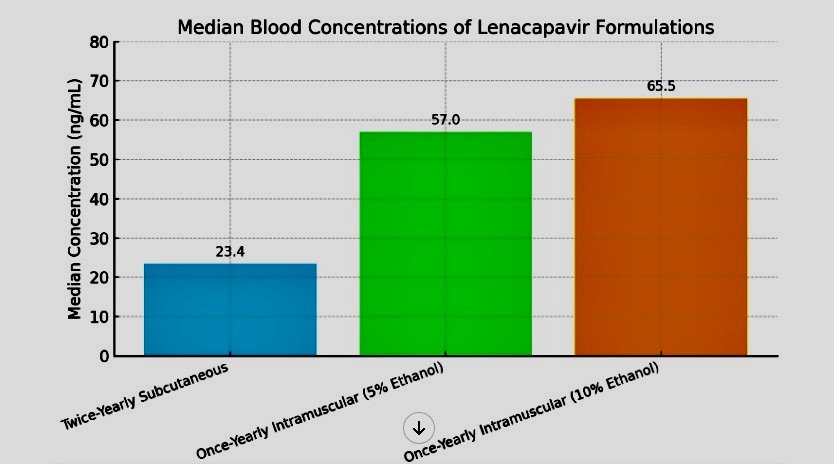
In the trial, 40 participants received intramuscular injections of Lenacapavir at a dose of 5 grams, delivered in formulations containing either 5% or 10% ethanol to reduce injection viscosity.
At the one-year mark, the median blood concentrations were 57 ng/mL and 65.5 ng/mL, respectively.
These levels significantly exceeded the 23.4 ng/mL achieved by twice-yearly subcutaneous injections at the six-month point in prior phase 3 studies.
Safety and Tolerability of Lenacapavir:
Both formulations of once-yearly lenacapavir were well tolerated. No grade 4 treatment-emergent adverse events were reported, and while some participants experienced grade 3 lab abnormalities, these were deemed unrelated to the drug.
The most common grade 3 adverse event was elevated low-density lipoprotein (LDL) cholesterol, which occurred in four participants who already had abnormal LDL levels at baseline.
Injection-site pain was a common but mild issue, substantially alleviated by pre-treatment with ice. The findings underscore the safety of lenacapavir’s intramuscular formulation, setting the stage for its progression to phase 3 studies.
Gilead’s Vision for HIV Prevention:
Gilead is preparing to launch phase 3 trials for the once-yearly formulation in the second half of 2025, with potential regulatory filings anticipated in 2027.
Dr. Jared Baeten, Gilead’s vice president of HIV clinical development, emphasized the unmet need for longer dosing intervals, as expressed by the scientific community and individuals affected by HIV.
The company’s choice of intramuscular administration for the annual dose reflects a strategic decision to optimize pharmacological performance and accommodate the larger drug volume required.
Notably, the researchers believe that future development may involve lower doses than the 5 grams used in the phase 1 study, based on the unexpectedly high drug concentration levels observed.
The Path Forward ...
Gilead’s phase 3 program will likely focus on a pharmacological endpoint rather than relying solely on infection rates, as the safety and efficacy benchmarks have already been established in earlier studies.
Additionally, oral loading doses may be incorporated into the regimen to achieve desirable initial plasma concentrations more quickly.
As the company moves forward, it is also awaiting an FDA decision on its twice-yearly lenacapavir PrEP formulation, which is under priority review with a decision expected by June 2025.
Currently approved under the brand name Sunlenca for treating multidrug-resistant HIV, lenacapavir’s potential expansion into prevention marks an exciting development in the fight against HIV/AIDS.
A New Era in HIV Prevention:
The prospect of a once-yearly PrEP option could revolutionize HIV prevention by offering unprecedented convenience and adherence benefits.
If successful, lenacapavir’s annual dosing regimen could become a cornerstone in reducing HIV transmission worldwide, addressing the ongoing challenge of long-term adherence to preventive therapies.
With phase 3 trials on the horizon, Gilead’s commitment to innovation in HIV care continues to inspire hope for a healthier future.







