BOTOX or Botulinum toxin is a neurotoxin that causes denervation by inhibiting the calcium-dependent acetylcholine release in the presynaptic neurons. It is used to treat the following conditions:
-
Treatment of patients with axillary sweating also called axillary hyperhidrosis in adults that do not respond to topical therapy.
- Therapy with BOTOX should be initiated only when secondary causes like hyperthyroidism have been ruled out..
- Other than in the axillary region, treatment for hyperhidrosis may have negative side effects like weakness.
-
Treatment for patients 16 years of age or older with cervical dystonia.
-
For the prevention of persistent migraines that last longer than four hours per day for at least 15 days each month.
-
For the treatment of adult lower limb spasticity.
-
For the treatment of overactive bladder and urge incontinence in patients who have a poor or inadequate response to anticholinergic medicines.
-
For the treatment of Strabismus and blepharospasm associated with dystonia including benign blepharospasm in patients older than 12 years of age.
-
For the treatment of Upper limb spasticity.
-
For the treatment of Urinary incontinence due to detrusor overactivity in patients with neurologic conditions like multiple sclerosis and spinal cord injury.
-
Patients with children who have a dynamic equinus foot deformity have cerebral palsy.
-
Off-Label Uses of BOTOX in Adults include:
- Achalasia
- Anal fissures
- Severe Raynaud phenomenon that is refractory to initial conventional therapy.
- Drooling of saliva or Sialorrhea
- Tardive dyskinesias
BOTOX use for cosmetic purposes
-
to treat Glabellar lines that are moderate to severe temporarily.
-
for the short-term treatment of forehead lines and moderate to severe wrinkling.
-
for the (temporary) treatment of eyelid lateral canthus lines that range from mild to severe.
BOTOX Dose in Adults
BOTOX Dose in various conditions:
- When initiating therapy, the lowest recommended dose should be used.
- The maximum cumulative dose should be less than 400 units per 3 months or 6 units/kg over 3 months
BOTOX Use in the treatment of Bladder dysfunction:
- Detrusor overactivity associated with neurologic conditions:
- A total of 200 units/30 mL (6.7 units/mL) were injected in the detrusor muscle during the course of 30 injections.
- The final injection should be followed by 1 mL of sterile Normal saline
- Re-treatment may be considered 12 weeks after the last dose.
- Overactive bladder:
- A maximum dose of 100 units may be administered in the detrusor muscle after 20 injections of 0.5 mL (10 units/mL) each.
- The final injection should be followed by 1 mL of sterile Normal saline
- Re-treatment may be considered 12 weeks after the last dose.
NOTE:
- To lower the risk of urinary tract infections, prophylactic antibiotics should be given 1–3 days before, the day of, and 1–3 days following the injection.
- Antiplatelets should be discontinued for at least 3 days before BOTOX administration.
Use in the treatment of Blepharospasm:
[caption id="attachment_5260" align="aligncenter" width="600"] Closeup of botox injection in wrinkle near eye[/caption]
Closeup of botox injection in wrinkle near eye[/caption]
- 1.25 to 2.5 units intramuscularly injected (into the medial and lateral pretarsal orbicularis oculi of the upper lid and lateral pretarsal orbicularis oculi of the lower lid).
- The following dose may be doubled up to a maximum of 5 units if the response is insufficient.
- The Cumulative dose according to the US labeling should not exceed 200 units in 30 days or 200 units in a 2-month period (according to the Canadian labeling).
Use in the treatment of Cervical dystonia:
[caption id="attachment_5261" align="aligncenter" width="220"] Injection BOTOX for cervical dystonia[/caption]
Injection BOTOX for cervical dystonia[/caption]
- The mean dose is 236 units divided among the affected muscles in patients previously treated with botulinum toxin (maximum dose per site is 50 units or less).
- Patients who have never received treatment before may receive a lesser dose.
- The patient's head and neck position, pain, muscle hypertrophy, responsiveness, and prior response to therapy, including any negative side effects, should all be taken into consideration while determining BOTOX dosage in sequence.
- 100 units should not be more than the entire dose injected into the sternocleidomastoid muscles.
- According to Canadian labeling:
- The effective BOTOX range of 200 - 360 units intramuscular has been used in clinical practice and should be administered at a minimum interval of 2 months.
Use in the treatment of Chronic migraine:
[caption id="attachment_5262" align="aligncenter" width="1024"] Migraine protocol - BOTOX injection[/caption] Administer 5 units/0.1 mL per site.
Migraine protocol - BOTOX injection[/caption] Administer 5 units/0.1 mL per site.
- The recommended dosage is 155 units administered as intramuscular injections in divided doses (into 31 different sites) bilaterally once every 12 weeks.
- The following are the recommended sites of administration:
- Corrugator:
- 5 units to each side (two sites)
- Procerus:
- 5 units (one site only)
- Frontalis:
- 10 units to each side (divided into two sites per side)
- Temporalis:
- 20 units to each side (divided into four sites per side)
- Occipitalis:
- 15 units to each side (divided into three sites per side)
- Cervical paraspinal:
- 10 units to each side (divided into two sites per side)
- Trapezius:
- 15 units to each side (divided into three sites per side)
- Corrugator:
Use in the treatment of Spasticity:
- Treatment should be individualized based on the severity, size of the muscle, the extent of the disease, location of the muscle, and response to prior treatment.
- The dose may be given again after three months or longer.
- Lower limb spasticity:
- Use the lowest effective dose and deliver 50 units/site or less in a series of sequential injections as follows::
- Flexor digitorum longus:
- 50 units divided into 2 sites.
- Flexor hallucis longus:
- 50 units divided into 2 sites
- The lateral head of Gastrocnemius:
- 75 units divided into 3 sites.
- The medial head of Gastrocnemius:
- 75 units divided into 3 sites.
- Soleus:
- 75 units divided into 3 sites
- Tibialis posterior:
- 75 units divided into 3 sites
- Flexor digitorum longus:
- Use the lowest effective dose and deliver 50 units/site or less in a series of sequential injections as follows::
- Upper limb spasticity:
- When administering the following dosage recommendations, the minimal advised dose should be used and 50 units/site or fewer should be used.:
- Adductor pollicis:
- 20 units - 1 site
- Biceps brachii:
- 100 - 200 units divided into 4 sites
- Flexor digitorum profundus:
- 30 - 50 units - 1 site
- Flexor digitorum sublimes:
- 30 - 50 units - 1 site
- Flexor carpi radialis:
- 12.5 - 50 units - 1 site
- Flexor carpi ulnaris:
- 12.5 - 50 units - 1 site
- Flexor pollicis longus:
- 20 units - 1 site
- Adductor pollicis:
- When administering the following dosage recommendations, the minimal advised dose should be used and 50 units/site or fewer should be used.:
Use in the treatment of Strabismus:
[caption id="attachment_5263" align="aligncenter" width="696"] Strabismus treatment with BOTOX injection[/caption] Note: Before BOTOX therapy, administer local anesthetic and ocular decongestant drops.
Strabismus treatment with BOTOX injection[/caption] Note: Before BOTOX therapy, administer local anesthetic and ocular decongestant drops.
- Vertical muscles and for horizontal strabismus less than 20 prism diopters:
- 1.25 to 2.5 units in any one muscle intramuscularly.
- Horizontal strabismus of 20 - 50 prism diopters:
- 2.5 - 5 units in any one muscle
- Persistent sixth nerve palsy of more than 1 month:
- 1.25 - 2.5 units in the medial rectus muscle.
- The patient must be reassessed after one to two weeks to assess the effect of the injection.
- Subsequent doses may be increased to twice the previous dose, up to the maximum recommended dose of 25 units (as a single injection for any one muscle).
- Subsequent injections must be delayed until the effects of the previous injections are present.
Use in the treatment of Primary axillary hyperhidrosis:
- 50 units intradermally per axilla.
- As described below in the "How to give BOTOX?" section, the injection location should be identified using conventional staining methods.
- Injections should be given in 0.1 to 0.2 mL aliquots, spaced 1 to 2 cm apart, at ten to fifteen different sites.
- When the clinical effect lessens, the dose may be given again.
BOTOX dose for Cosmetic use:
- Reduction of forehead lines
- Inject into 5 injection sites of 0.1 mL (4 units) intramuscular for a total dose of 0.5 mL (20 units) in the frontalis muscle administered at an interval of at least 3 months.
- Glabellar lines should be treated in conjunction with the forehead lines to reduce the risk of brow ptosis.
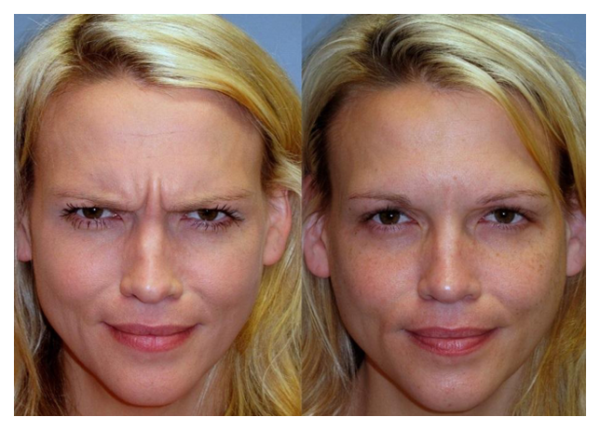
- [caption id="attachment_5264" align="aligncenter" width="644"]
 BOTOX for glabellar lines before and after[/caption]
BOTOX for glabellar lines before and after[/caption] - Reduction of glabellar lines:
- For a total dose of 0.5 mL or 20 units, which should be taken no more frequently than every 3 to 4 months, inject intramuscularly into each of the five sites 0.1 mL, or four units, twice into each corrugator muscle, and once into the procerus muscle.
- Reduction of lateral canthus lines:
- For a total dose of 0.6, intramuscularly administer 0.1 mL, or 4 units, to the lateral orbicularis oculi muscle at 3 injection spots on each side.
BOTOX dose in Children:
Use for treating Blepharospasm:
- Children older than 12 years and Adolescents:
- The medial and lateral pretarsal orbicularis oculi of the upper lid's and the lateral pretarsal orbicularis oculi of the lower lid were both intramuscularly injected with 1.25 to 2.5 units.
- The dose may be raised up to a maximum dose of 5 units per location if the response lasts less than two months or is insufficient.
- In a 30-day period, the maximum cumulative dose should not exceed 200 units.
Use for treating Cervical dystonia:
- Adolescents 16 years of age or older:
- A maximum of 50 units/site, or 236 units total, are administered intramuscularly to the afflicted muscles as the mean dose.
- The first dose of treatment should be reduced, and successive doses should be modified according to the patient's head and neck position, muscle hypertrophy, pain localisation, responsiveness, and history of negative responses.
- In order to reduce the incidence of dysphagia,
Use in the treatment of Strabismus in patients less than 12 years of age:
Note: Administer a local anesthetic and a decongestant drop before BOTOX injection.
- Infants and Children 2 months to 11 years of age:
-
Horizontal or vertical deviations of less than 20 prism diopters:
- Patients weighing 6 kgs or more:
- 1.25 units into any one muscle
- Patients weighing 6 kgs or more:
-
Horizontal or vertical deviations 20 - 50 prism diopters:
- Patient weighing less than 6 kgs:
- One unit into any one muscle
- Patient weight 6 - 9 kg:
- 1.25 units into any one muscle
- Patient weight 10 - 12.5 kg:
- 1.25 - 1.75 units into any one muscle
- Patient weighing more than 12.5 kgs:
- 1.25 - 2.5 units into any one muscle
- Patient weighing less than 6 kgs:
-
Persistent Sixth nerve palsy of one month or more in duration:
- Patient weighing less than 6 kgs:
- One unit into the medial rectus muscle
- Patient weighing 6 kgs or more:
- 1.25 units into the medial rectus muscle
- Patient weighing less than 6 kgs:
-
- Children more than 12 years and Adolescents:
- Vertical muscles and for horizontal strabismus of less than 20 prism diopters:
- 1.25 - 2.5 units intramuscular into any one muscle
- Horizontal strabismus 20 - 50 prism diopters:
- 2.5 - 5 units into any one muscle
- Persistent sixth nerve palsy of 1 month or more:
- 1.25 - 2.5 units into the medial rectus muscle
- The patient must be reevaluated 7 to 14 days after each injection (to assess the effect of that dose).
- Up to a maximum dose of 25 units in a single injection, subsequent doses may be doubled after the effects of the preceding dose have worn off.
- Vertical muscles and for horizontal strabismus of less than 20 prism diopters:
Use for treating Spasticity associated with cerebral palsy:
- Children older than18 months and Adolescents:
- Upper extremity:
- 0.5 to 2 units/kg for each muscle, with a maximum dosage per visit of fewer than 13 units/kg. (Reported dosage range is 0.3 to 4 units/kg per muscle, with a 400 unit maximum total dose)
- Lower extremity:
- To get a cumulative dose of 200 units over a three-month period, the total dosage range is 4 to 8 units/kg.
- For diplegia:
- Six units/kg split across the afflicted limbs made up the initial total dosage.
- For equinus foot:
- At two different locations in the gastrocnemius muscle of the afflicted lower limb, a total dosage of 4 units/kg is injected.
- Upper extremity:
Pregnancy Risk Factor: C
- Fetal harm has been reported with its use including decreased fetal body weight and delayed ossification.
Use during breastfeeding:
- It should be used with caution in lactating women as excretion of the drug into breast milk is not known.
BOTOX Dose in Renal Disease:
- The company does not advise individuals with renal disorders to change their dosage.
BOTOX Dose in Liver Disease:
- The manufacturer has not recommended any dose adjustment in patients with hepatic impairment.
Negative effects often start to show within a week and might linger for several months.
Common side Effects Of BOTOX (ona botulinum toxin A) Include:
- Bladder dysfunction:
- Genitourinary:
- Urinary retention
- Increased postvoid residual urine volume
- Urinary tract infection
- Bacteriuria
- Genitourinary:
- Cervical dystonia:
- Respiratory:
- Upper respiratory tract infection
- Central nervous system:
- Headache
- Neuromuscular & skeletal:
- Neck pain
- Gastrointestinal:
- Dysphagia
- Respiratory:
- Primary axillary hyperhidrosis, strabismus, forehead lines, glabellar lines, lateral canthal lines, and blepharospasm:
- Ophthalmic:
- Vertical deviation of eyes
- Blepharoptosis
- Ophthalmic:
Less Common Side Effects Of Ona Botulinum Toxin A Include:
- Bladder dysfunction:
- Gastrointestinal:
- Constipation
- Central nervous system:
- Myasthenia
- Abnormal gait
- Falling
- Immunologic:
- Antibody development
- Genitourinary:
- Dysuria
- Hematuria
- Neuromuscular & skeletal:
- Muscle spasm
- Gastrointestinal:
- Cervical dystonia:
- Central nervous system:
- Dizziness
- Numbness
- Drowsiness
- Speech disturbance
- Hypertonia
- Local:
- Injection site reaction:
- Soreness
- Injection site reaction:
- Gastrointestinal:
- Nausea
- Xerostomia
- Immunologic:
- Antibody development
- Respiratory:
- Cough
- Dyspnea
- Flu-like symptoms
- Rhinitis
- Neuromuscular & skeletal:
- Back pain
- Stiffness
- Asthenia
- Ophthalmic:
- Blepharoptosis
- Diplopia
- Miscellaneous:
- Fever
- Central nervous system:
- Chronic migraines:
- Cardiovascular:
- Hypertension
- Local:
- Pain at the injection site
- Central nervous system:
- Facial paresis
- Exacerbation of migraine headache
- Headache
- Ophthalmic:
- Blepharoptosis
- Neuromuscular & skeletal:
- Musculoskeletal pain
- Myalgia
- Neck pain
- Myasthenia
- Stiffness
- Muscle spasm
- Respiratory:
- Bronchitis
- Cardiovascular:
- Lower limb spasticity:
- Neuromuscular & skeletal:
- Arthralgia
- Back pain
- Myalgia
- Local:
- Pain at the injection site
- Respiratory:
- Upper respiratory tract infection
- Neuromuscular & skeletal:
- Upper limb spasticity:
- Gastrointestinal:
- Nausea
- Central nervous system:
- Fatigue
- Respiratory:
- Bronchitis
- Neuromuscular & skeletal:
- Limb pain
- Myasthenia
- Gastrointestinal:
- Reduced glabellar lines, strabismus, main axillary hyperhidrosis, forehead, glabellar, and lateral canthal lines, as well as blepharospasm:
- Dermatologic:
- Skin tightness
- Diaphoresis
- Pruritus
- Skin rash
- Central nervous system:
- Headache
- Facial pain
- Facial paresis
- Anxiety
- Dizziness
- Pain
- Gastrointestinal:
- Nausea
- Local:
- Injection site reaction:
- Soreness
- Pain
- Hemorrhage
- Injection site reaction:
- Immunologic:
- Antibody development
- Infection:
- Infection
- Ophthalmic:
- Diplopia
- Ectropion
- Dry eye syndrome
- Eyelid edema
- Keratitis
- Lacrimation
- Eye irritation
- Eyelid entropion
- Superficial punctate keratitis
- Neuromuscular & skeletal:
- Asthenia
- Back pain
- Neck pain
- Respiratory:
- Flu-like symptoms
- Pharyngitis
- Miscellaneous:
- Fever
- Dermatologic:
Contraindication to BOTOX (ona botulinum toxin A) include:
- Allergic reactions to the drug or any component of the formulation.
- Infection at the injection site.
- Patients with a UTI or postvoid residual urine volume of more than 200 m for Intradetrusor injection
Warnings & Precautions
- Allergic reactions:
- There might be dyspnea, urticaria, soft tissue edoema, anaphylactic responses, and serum sickness.
- Antibody formation:
- Repeated and higher doses result in loss of efficacy due to antibody formation.
- Autonomic dysreflexia:
- After receiving a BOTOX injection intradetrusor, individuals with spinal cord injuries may have dysautonomia.
- Patients may develop resting tachycardia, fluctuating Blood Pressure readings, sweating, and may at times present with hypertensive crisis.
- Cardiovascular events:
- Patients with risk factors for cardiovascular diseases and those with preexisting heart disease may develop cardiac arrhythmias and myocardial infarction.
- Dysphagia:
- Patients with smaller neck muscles, injections into the levator scapulae, and those with bilateral injections are at risk of dysphagia.
- Dysphagia may be severe enough to require alternative feeding methods and may put the patients at risk of aspiration.
- Hematologic:
- Patients with a bleeding disorder and those on anticoagulants should use the drug with caution.
- Ophthalmic effects:
- When the orbicularis oculi muscle is injected, patients may have dry eyes, discomfort, photophobia, ocular pain, and altered vision.
- Respiratory effects:
- Upper respiratory tract infections like bronchitis can occur in patients undergoing treatment for upper or lower limb spasticity.
- Systemic toxicity: [US Boxed Warning]:
- Dysphagia and life-threatening respiratory failure may occur as a result of the systemic spread of the drug.
- In the hours or weeks following the injections, patients may also have diplopia, impaired vision, ptosis, asthenia, dysarthria, dysphonia, widespread muscular weakness, and incontinence.
- Urinary retention:
- Urinary retention can occur in patients receiving treatment for an overactive detrusor or an overactive bladder.
- Treatment should be offered only if the patient is willing for post-treatment catheterization.
- Urinary tract infections:
- An increase in the incidence of UTIs has been reported with its use, following intradetrusor administration.
- Patients who have had 2 or more episodes of UTIs in the past 6 months and diabetic patients are especially at risk.
- Episodic migraines:
- Efficacy of treatment in patients with less than 14 headaches per month has not been established.
- Neuromuscular disease:
- Patients with neuromuscular diseases such myasthenia gravis, Lambert-Eaton myasthenic syndrome, and motor neuron disease may experience a worsening of their symptoms.
- Ocular diseases:
- Exposure of the cornea When treating blepharospasm, decreased blinking may result in persistent corneal epithelial defects and ulceration.
- When treating strabismus, needle penetration into the orbit might cause retrobulbar haemorrhage.
- Glaucoma patients should use the medication with care.
- Respiratory disease:
- Patients who already have a respiratory condition should use the medication extremely cautiously since it may deteriorate the supporting muscles for breathing.
Monitor:
- Detrusor overactivity:
- two weeks after therapy, and then at regular intervals for up to twelve weeks, measure the volume of postvoid residual (PVR) urine.
- When the PVR urine volume is greater than 200 mL, catheterization should start and continue until the PVR is less than 200 mL.
How to administer BOTOX (ona botulinum toxin A)?
-
Administration of intradetrusor for the treatment of neurologic condition-related bladder dysfunction, including hyperactive detrusors and overactive bladder:
- Prophylactic antibiotic therapy (other than aminoglycosides) is advised to be given one to three days prior to, the day of, and for one to three days following BOTOX administration.
- Antiplatelet medication should be ceased for at least three days prior to its administration.
- In order to prepare the bladder for a local anaesthetic injection prior to therapy, the bladder must first be drained and irrigated with normal saline.
- Saline should be injected into the bladder to improve vision (avoid overdistention).
- Prime the syringe with roughly 1 mL of reconstituted BOTOX before injecting it to get rid of any air bubbles.
- To deliver BOTOX, use a flexible or rigid cystoscope.
- Place the needle 2 mm into the detrusor using the required volume and without touching the trigone.
- Approximately 1 centimetre should separate each injection.
- Drain the saline from the patient's bladder after the surgery, and continue to watch them for at least 30 minutes.
- In situations of the overactive bladder, a spontaneous void should also occur before the patient leaves the clinic.
-
Blepharospasm:
- Use a needle that is 27 or 30 gauge.
- Avoid injecting close to the medial lower lid and the levator palpebrae superioris.
- To avoid ecchymosis, pressure should be administered to the injection site.
-
Cervical dystonia:
- For the deep muscles, use a longer 22 gauge needle and a larger 25, 27, or 30 gauge needle. To pinpoint the affected muscles' locations, electromyography may be utilised.
-
Chronic migraines:
- 5 units per 0.1 mL should be injected into each of the 31 locations using a 0.5 inch, 30 gauge needle.
Spasticity as in cerebral palsy and related conditions:
- Use a needle between 23 and 26 gauge.
- Administer the medication to the afflicted limb's medial and lateral gastrocnemius muscles.
-
Spasticity in the upper or lower limb:
- For the deeper muscles, use a longer 22 gauge needle instead of a shorter 25, 27, or 30 gauge needle.
- The affected muscles can be identified using nerve stimulation or electromyography.
-
Strabismus injections:
- When using BOTOX to treat strabismus, electromyographic guidance or surgical exposure are necessary.
- The placement of the medication into the target muscle may be guided by the electrical activity detected at the injection needle's tip.
- A topical anaesthetic and an eye decongestant should be administered prior to the injection.
- 0.05 to 0.15 ml may be injected per muscle, however, the majority of patients will need further doses.
-
Primary axillary hyperhidrosis:
- At a 45° angle, inject intradermally to a depth of roughly 2 mm.
- Prior to the injection, the injection location should be designated and characterised using common staining methods such as Minor's Iodine-Starch Test.
- Instructions for Test:
- The patient should shave their underarms prior to the test and wait 24 hours before applying deodorants or antiperspirants.
- The patient should take a 30-minute nap and abstain from hot liquids.
- After drying, an iodine solution should be administered to the underarm region.
- The starch powder should be sparingly dusted over the surface once it has dried.
- In approximately 10 minutes, the sweating area should be a deep blue-black hue when the extra powder is blown off.
Administration of BOTOX for cosmetic purposes
-
Reduction of glabellar lines:
- Use a needle between 30 and 33 gauge.
- inject into every one of the five places (2 injections in each corrugator muscle and 1 injection in the procerus muscle).
- Utilize the smallest volume feasible and stay away from the levator palpebrae superioris while injecting.
-
Reduction of forehead lines:
- Use a needle of 30 to 33 gauge.
- Five locations in the frontalis muscle should get injections.
-
Reduction of lateral canthal lines:
- Make use of a 30 to 33-gauge needle.
- The bevel tip of the needle should be injected up and away from the eye.
- Inject the lateral orbicularis oculi muscle at 3 places on each side (a total of 6 injection points).
Mechanism of action of BOTOX (Botulinum toxin A):
- The neurotoxin BOTOX is created by the bacterium Clostridium botulinum and is sometimes referred to as Ona botulinum toxin A (formerly known as botulinum toxin type A).
- The anaerobic spore-forming bacterium Clostridium botulinum produces the botox toxin, which prevents the calcium-dependent release of acetylcholine in the presynaptic membrane of the neuromuscular junction in humans. Denervation is the term for this disorder.
- Muscle inactivation lasts as long as junction plates do not develop from new fibrils that emerge from the nerves.
- By blocking the release of acetylcholine, botox injections intratumorally alter the efferent pathways of detrusor activity.
- When botox is injected intradermally, sweat glands are momentarily rendered inactive, which lessens localised perspiration.
Improvement in the symptoms may be noted as follows:
- Improvement in blepharospasm is noted in 3 - 4 days.
- Improvement in Cervical dystonia is noted in 2 weeks.
- Improvement in Detrusor overactivity is noted in about 2 weeks.
- Improvement in the reduction of glabellar lines may be seen in 1 - 2 days
- Improvement in Spasticity in patients with focal and cerebral palsy may be noted in 2 weeks or less time.
- Improvement in strabismus may be observed in 1 - 2 days
Duration of action of BOTOX:
It is effective for the following duration:
- Blepharospasm:
- 3 to 4 months
- Detrusor overactivity associated with the neurologic condition:
- 42 - 48 weeks
- Cervical dystonia:
- 3 - 4 months
- Primary axillary hyperhidrosis:
- For a mean duration of 201 days.
- Spasticity:
- 3 - 3.5 months
- Reduction of glabellar lines:
- 3 - 4 months
- Strabismus:
- 2 - 6 weeks
- 2 - 6 weeks
It is not absorbed when administered Intramuscularly at the recommended doses.
The Time to peak effect depends on the indication:
- Focal Spasticity:
- 4 to 6 weeks
- Cervical dystonia:
- ~6 weeks
- Strabismus:
- Within the first week
- Blepharospasm:
- 1 to 2 weeks
Botox (Botulinum toxin type A) international brands:
- Botox
- Lanzox
- Vista
BOTOX brand in Pakistan:
BOTOX injection (Botulinum Toxin Type A)