Orencia (Abatacept) is a non-TNF (tumor necrosis factor) Biologic medicine. It has a novel mechanism of action (MOA) than the other disease-modifying antirheumatic drugs (DMARDs).
Orencia MOA - How Abatacept works?
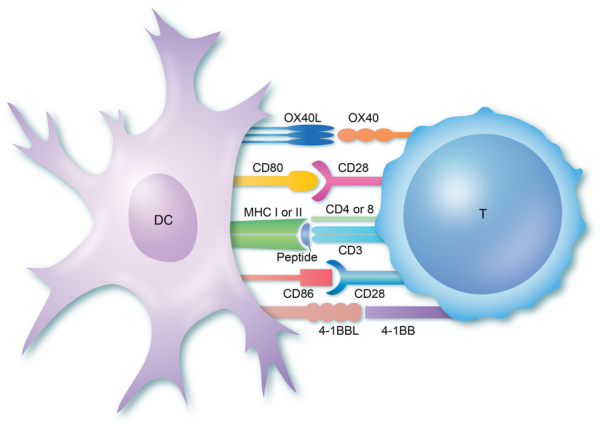
Before discussing how abatacept functions as an immunomodulator, one should know how the T - lymphocytes are activated. T- cells (or T-lymphocytes) are activated by antigen-presenting cells. The first step is the binding of antigen to the MHC molecule of the antigen-presenting cell. This is followed by the tri-molecular complex formation when the antigen and the antigen-presenting cell binds to the receptors on the T-cells. [caption id="attachment_9672" align="aligncenter" width="600"] T Cell Activation step 1: Formation of the tri-molecular complex[/caption] This is followed by the next important step of T-cell activation and that is the binding of cell surface co-stimulatory molecules. There are two important co-stimulatory molecules that activate the process:
T Cell Activation step 1: Formation of the tri-molecular complex[/caption] This is followed by the next important step of T-cell activation and that is the binding of cell surface co-stimulatory molecules. There are two important co-stimulatory molecules that activate the process:
-
CD 28 which is an activation receptor and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) is an inhibitory receptor that binds to CD80 or CD86.
-
CD40 binds to CD40 ligand (also known as CD40L, CD154, or gp39).
 Orencia MOA Step - 2: T-cell activation by co-stimulatory signals[/caption] These co-stimulatory molecules are responsible for cellular activation and T-cells dependent B-cells stimulation.
Orencia MOA Step - 2: T-cell activation by co-stimulatory signals[/caption] These co-stimulatory molecules are responsible for cellular activation and T-cells dependent B-cells stimulation.
How abatacept acts (Orencia MOA)?
Abatacept (Orencia) is a soluble protein that is made by the fusion of the Fc portion of the IgG-1 molecule and the extracellular domain of CTLA4 (cytotoxic T lymphocyte-associated antigen 4). CTLA4-Ig, (Abatacept), binds CD80(B7-1), and CD86 (B7-2), on antigen-presenting cell. This is a competitive inhibitor for the CD28-B7 costimulatory interaction (CD80 and CD86). Because soluble CTLA4Ig (Abatacept), binds to CD80, (B7-1), and CD86 (B7-2), T cells are unable to receive the second important activation signal via CD28.
Orencia (Abatacept) use in autoimmune rheumatic diseases:
Orencia (Abatacept) is approved by the FDA for the treatment of moderately to severely active Rheumatoid Arthritis who have an inadequate response to one or more of the disease-modifying anti-rheumatic drugs (DMARDs) or TNF (Tumor necrosis factor) inhibitors. It is also being used in patients with Psoriatic arthritis and Juvenile Idiopathic arthritis who have a moderately to severely active disease despite the use of DMARDs or other biologics. It is also being used off-label in patients with proliferative lupus nephritis. The concomitant use of other biological DMARDs (including Interleukin 1 inhibitors like anakinra and TNF inhibitors) should be avoided.